Recent Highlights
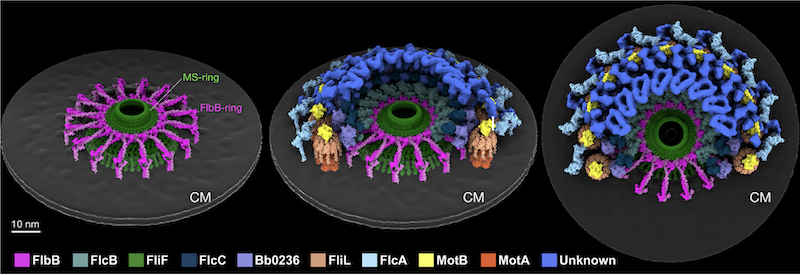
FlbB forms ring for assembly and motility
For this publication, the authors utilized SEC-SAXS data collected at SIBYLS beamline 12.3.1, along with SAXS model fitting, to confirm and further analyze the proteins of interest. Beamline scientist Michal[…]
Read moreSIBYLS team enjoy Kayaking at Elkhorn Slough Reserve
The SIBYLS group (and Halbach award winners) enjoy a well-deserved day out kayaking at one of California’s largest protected coastal salt marshes which provides habitat for a fantastic diversity of[…]
Read more2025 SAXS Workshop a Big Success
We had a fantastic turnout for our one-day Zoom-based SAXS workshop, with 37 researchers from 25 unique labs across the U.S. in attendance. The user project session and the breakout[…]
Read moreRegister for SAXS Workshop
We are excited to announce a zoom-based SAXS workshop designed to help ensure the success of both past and future projects. This workshop is open to all researchers, from those[…]
Read more